CIED Remote Monitoring
Streamline Workflows and Improve Outcomes with Alert-Based Monitoring Request a demo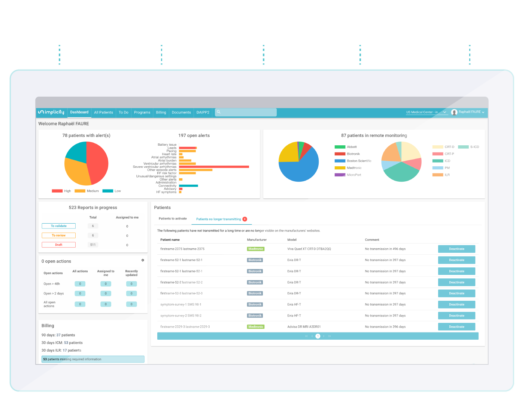
The universal platform to improve patient care and optimize revenue
Implicity’s alert-based Cardiac Remote Monitoring platform provides a comprehensive solution for health professionals seeking accurate and efficient cardiovascular monitoring. Our AI-driven technology transforms and aggregates data from all cardiac electronic implantable devices, regardless of vendor, to meet your specific needs.
Designed by an Electrophysiologist, our platform addresses the exact pain points experienced in daily practice. With Implicity, you can easily diagnose, rehabilitate, and surveil your cardiovascular patients.
Get Started Now

Cloud-Based platform

Intuitive interface

Available in less than 24 hours

Accessible everywhere
Unique Features and Core Capabilities
ILR ECG Analyzer1
FDA-cleared algorithm
Utilize AI to reduce the number of false positives by 79% when analyzing ECG recordings from patients implanted with Medtronic ILRs, while maintaining a sensitivity of 99%.2
Patient Connectivity Management
Reconnect 28%3 of patients two days after they disconnect using automated text message reminders.
InLink
Streamline your In-Clinic data workflow.
AF Alert Management4
Detect clinically relevant events proven to lead to an 85% decrease in unnecessary AF alerts compared to alerts transmitted by a cardiac implantable electronic device (CIED).
Dynamic Billing Engine
Achieve 90%+ billing compliance5 with optimized scheduling and auto-generated reports.
1. FDA cleared Class II medical device and CE marked Class I (under MDD) medical device; see the instructions for use for more information. 2. Read Academic Group Article 6393406 3. Durand, Julien, et al., Using Technology to Improve Reconnection to Remote Monitoring in Cardiac Implantable Electronic Device Patients, Cardiovascular Digital Health Journal, Volume 0, Issue 0 Published: November 23, 2023 DOI. 4. IM009: 2021. Manufacturer: Implicity. IM009 is a software as a medical device (SaMD) intended to be used as an adjunct of a remote monitoring platform to follow-up with target population patients. 5. Jay Ganji, MD, FACC, Cardiologist, Piedmont Cardiovascular, November 2022.
